
Top B.Pharm College in Mangalore

What's on NITTE?
National Youth Day
12
Jan 2024
The NSS and Youth Red Cross units of NGSM Institute of Pharmaceutical Sciences organized a....
Read more...
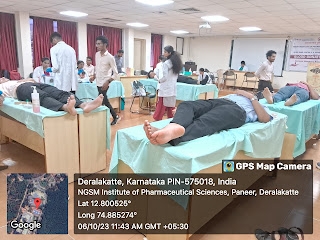








The NSS and Youth Red Cross units of NGSM Institute of Pharmaceutical Sciences organized a....
Read more...







